Transforaminal Lumbar Interbody Fusion with Silicon Nitride Ceramic Spacers: A Follow-Up Case Report of Two Patients
Abstract
Jim A Youssef1, Sue Lynn Myhre and B. Sonny Bal
Study design: Case Report
Objective: The objective of this study was to evaluate the performance of the Amedica – Transforaminal Lumbar Interbody Fusion (TLIF) device in two patients with a history of Posterior Lumbar Interbody Fusion (PLIF).
Methods: Two patients who underwent a TLIF procedure with posterior spinal instrumentation using the Valeo® Silicon Nitride ceramic (Si3N4) interbody TLIF implant were reviewed. At 1-year follow up, clinical and radiographic outcomes using CT scans and dynamic x-rays were evaluated.
Results: CT scans and dynamic x-rays at one year demonstrated solid interbody fusion and solid posterolateral fusion in both patients. Bone appeared to be well formed to the Valeo® ceramic implant as well as through and behind the implant in the interbody space. Additionally, there was no evidence of subsidence, migration or implant fracture/failure.
Conclusions: Solid bony fusion was demonstrated on dynamic x-rays and CT scans at 1-year follow up, which may be due to the proprietary nature of the ceramic that promotes bony growth on the implant as well as through the implant, and the better imaging compatibility of the Valeo® ceramic implant. These preliminary results suggest that Valeo® may be an appealing substitute for use in a TLIF procedure instead of a standard titanium, PEEK (polyetheretherketone) or carbon fiber graft. Well-designed studies with large sample sizes are needed to confirm the capabilities and performance of the Valeo® ceramic implant.
Keywords
Lumbar Interbody Fusion, Ceramic Interbody Implant; Silicon Nitride (Si3N4), Transforaminal Lumbar Interbody Fusion (TLIF), Posterior Lumbar Interbody Fusion (PLIF), PEEK (polyetheretherketone), Silicon Nitride PEEK, Poly(Aryl-EtherEther-Ketone), Titanium; Cage
Introduction – Transforaminal Lumbar Interbody Fusion
When non operative actions for degenerative spinal disorders fall flat, surgical intervention frequently becomes the popular choice of treatment. Although the class of pathology matters, the surgical operation will generally include an arthrodesis procedure. The gravity of a successful initial fusion surgery is intensified not only by the growing number of arthrodesis procedures, but also by the rising costs for revisal operations. Additionally, fusion success appears to have an impact on patient reported outcomes [1].
Historically, reports of transforaminal lumbar interbody fusion (TLIF), first described by Blume [2] and popularized by Harms and Rolinger [3], reflect good clinical and radiographic outcomes [4-8]. In the TLIF procedure, a structural support (allograft bone or assorted cage designs) is placed within the middle or anterior aspect of the disc space via a posterolateral transforaminal route accompanied by pedicle screw fixation [5,8,9]. Many interbody grafts have been created that not only focus on restoring disc height but also maintain lordosis through the vertebral segment, create distraction and restore the normal weight distribution within the anterior column [8]. Considering the advantageous features of the TLIF technique, the design of the synthetic interbody graft chosen for the procedure may potentially affect the radiographic success of the procedure.
Originally, titanium cages were placed in the interbody space during a TLIF procedure [5]. These cages lead to subsidence through the vertebral body endplates, especially in osteoporotic patients, because the modulus of elasticity for titanium is much greater than bone [5]. Postoperative imaging was also challenging with the titanium interbody implants as the titanium results in significant image distortion making fusion determination challenging. With the problems associated with the use of metal cages, nonresorbable polymers, such as PEEK (polyetheretherketone) or carbon fiber reinforced PEEK (CFRP), were developed since these polymers exhibited similar mechanical properties to that of bone [5,10]. Yet, a large case series found that the rates of collapse, slippage and graft migration associated with the use of CFRP and PEEK cages were found to occur at rates of 3-10% [5,11]. Therefore, there was a clear need to identify a better material for spinal fusions.
The Valeo® (Valeo® TL VBR Lumbar Spacer, Amedica Corporation, Salt Lake City, UT) silicon nitride ceramic implant is composed of micro-composite ceramic with a surface that mimics cancellous structure. Valeo® is advertised to mimic the structure of bone by incorporating both a dense load-bearing component (fracture resistant) and a porous component, coupled with a surface texture, to promote bone attachment, more than traditional metal implants such as PEEK and titanium. Along with the increased bone growth properties (growth onto the implant as well as through the implant), and due to its composition the graft should also have better compatibility with surgical and diagnostic imaging techniques, which is vital for intraoperative placement of the graft. However, at this time, there is a lack of published data on the performance of the Valeo® (Valeo® TL VBR Lumbar Spacer, Amedica Corporation, Salt Lake City, UT) ceramic implant in lumbar fusions.
This case study reviews the performance of the Amedica – TL fusion device in posterior transforaminal interbody spinal fusion by describing the clinical and radiographic outcomes of two patients with one year follow up.
Case Report 1 – Transforaminal Lumbar Interbody Fusion Device
History and physical examination
A 47-year-old, non-osteoporotic woman reported back pain and weakness in her legs. The patient was diagnosed with lumbar spinal stenosis at L4-L5 with neurogenic claudication and axial low back pain at L4-L5. A neurological deficit was noted at her extensor hallucis longus bilaterally (4+/5).
Preoperative radiographic imaging
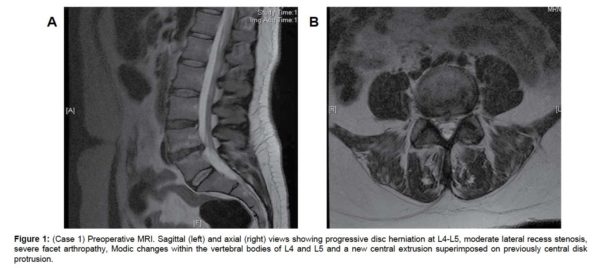
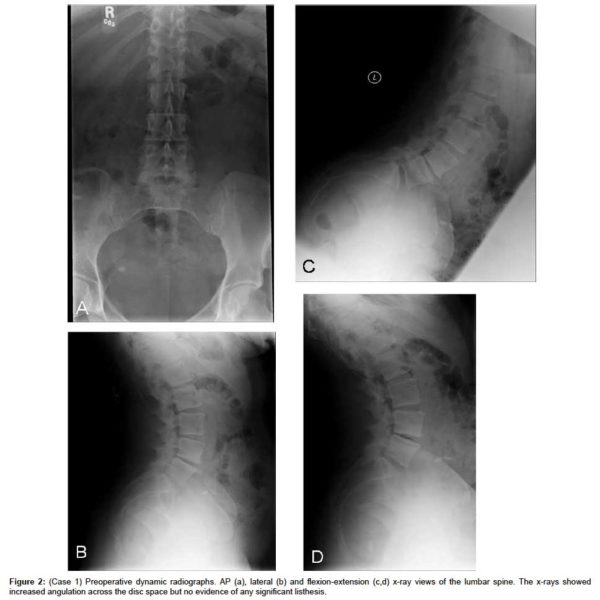
The MRI showed progressive disc herniation at L4-L5, moderate lateral recess stenosis, severe facet arthropathy, and Modic type 2 changes within the vertebral bodies of L4 and L5 (Figure 1a). The axial MRI demonstrated a new central extrusion superimposed on a previously central disc protrusion (Figure 1b). Anterior-posterior (AP), lateral and flexion-extension x-rays showed increased angulation across the disc space but no evidence of any significant listhesis (Figure 2(a & d), respectively).
Preoperative management
The patient was treated conservatively with physical therapy and oral medications. Due to the lack of improvement, treatment proceeded with diagnostic and therapeutic bilateral L5 transforaminal epidural injections. The injections were repeated in 2 months with no resolution of pain. As a result, surgery was recommended.
Operative management
A standard decompression, including laminectomy and facetectomy, and posterior spinal fusion (PSF) with instrumentation and TLIF with application of a Valeo® (Valeo® TL VBR Lumbar Spacer, Amedica Corporation, Salt Lake City, UT) silicon nitride ceramic implant was performed. Bone morphogenetic protein (Medtronic INFUSE® Bone Graft, Minneapolis, MN) was placed in the ceramic implant. Bone marrow aspirate, allograft, and local autograft harvested from lamina and spinous processes were placed posterior to the implant and in the disc space.
Postoperative course
Length of stay was two days in the hospital without complications. Postoperative x-rays demonstrated excellent placement of the implants, excellent restoration of the disk space height and adequate placement of the pedicle screws (Figure 3). At two-weeks postoperatively, all preoperative symptoms were resolved, and no neurological deficits were present, and the patient was walking over two miles a day. At eight-weeks postoperatively, the patient was enrolled into six weeks of physical therapy, and continued to do well clinically.
At one-year follow-up, bone appeared to be molded to the Valeo® implant (Valeo® TL VBR Lumbar Spacer, Amedica Corporation, Salt Lake City, UT), as well as through and behind the implant in the interbody zone. The patient demonstrated an anterior grade I fusion, and a posterior grade II fusion via x-rays [12] (Figure 4). The CT also displayed fusion based on Brantigan’s et al. [13] definition; that is, “the level was regarded as fused as there was radiographic evidence of bone bridging the disc space with no lucency” (Figure 5). Subsidence was deemed a grade zero [14]. Post-operative imaging was not distorted by the interbody implant, thus making fusion and subsidence assessments predictable.
Case Report 2 – TLIF Device
History and physical examination
A 77-year-old, non-osteoporotic female described low back pain and bilateral leg pain, left greater than right. The patient was diagnosed with lumbar spinal stenosis L4-L5 and disc space collapse L4-L5. She had no neurological deficits preoperatively.
Preoperative radiographic imaging
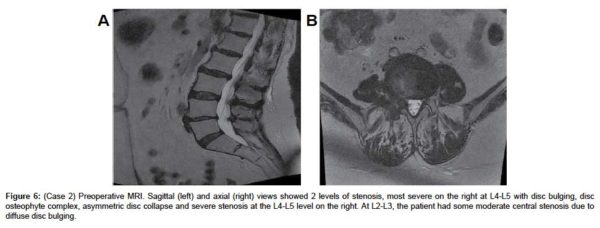
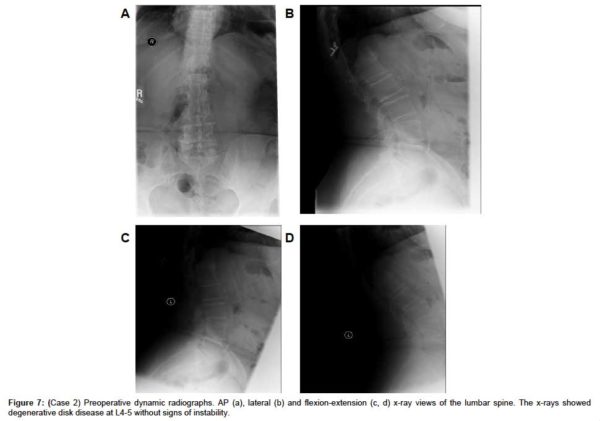
A sagittal MRI scan showed L4-L5 disc desiccation (Figure 6a). An axial MRI image of L4-L5 demonstrated foraminal stenosis, most severe on the right at L4-L5 with disc bulging, disc osteophyte complex, asymmetric disc collapse and severe stenosis on the right (Figure 6b). AP, lateral and flexion-extension x-rays showed degenerative disk disease at L4-5 without signs of instability (Figure 7(a & d), respectively).
Preoperative management
The patient was treated conservatively with physical therapy and oral medications. Due to the lack of improvement, treatment proceeded with diagnostic and therapeutic bilateral L5 transforaminal epidural injection. The patient failed non-operative efforts and was offered operative intervention.
Operative management
Similar to the first case report, the patient underwent a standard decompression (laminectomy and facetectomy), PSF with instrumentation and TLIF with application of a Valeo® (Valeo® TL VBR Lumbar Spacer, Amedica Corporation, Salt Lake City, UT) silicon nitride ceramic implant. Bone morphogenetic protein (Medtronic INFUSE® Bone Graft, Minneapolis, MN) was placed in the ceramic implant. Bone marrow aspirate, allograft and local autograft harvested from the lamina and spinous processes were placed posterior to the implant and in the disc space.
Postoperative course
Length of stay was two days in the hospital without complications. At two weeks postoperatively, the patient had some mild left leg pain, which decreased gradually. However, no neurological deficits were noted. The patient was treated with a brace for eight weeks postoperatively and started physical therapy shortly after being weaned off the brace. Postoperative x-rays demonstrated excellent placement of the implants, and no hardware loosening, failure or graft extrusion (Figure 8).
At 14 months, like case 1, bone seemed to be effectively formed to the Valeo® implant (Valeo® TL VBR Lumbar Spacer, Amedica Corporation, Salt Lake City, UT), as well as through and dorsal to the cage in the interbody space. Per x-rays, the patient demonstrated grade I fusions both anteriorly and posteriorly [12] (Figure 9). Also comparable to case 1, the CT displayed fusion success “as there was radiographic evidence of bone bridging the disc space with no lucency” [13] (Figure 10). Subsidence was judged to be a grade zero [14]. There was no distortion of the imaging due to the implant.
Discussion
The present case study aimed to assess the efficacy of the Amedica – Transforaminal Lumbar Interbody Fusion device in two patients with a history of posterior interbody spinal fusion. We found that the Valeo® (Valeo® TL VBR Lumbar Spacer, Amedica Corporation, Salt Lake City, UT) silicon nitride ceramic implant demonstrated solid bony growth on both the implant and through the implant in both patients on dynamic x-rays and CT scans at 1-year follow up. The post-operative imaging was not distorted by the interbody implant, making fusion assessment predictable, and there was no evidence of subsidence.
Most ceramics are attributed with osteointegration and osteoconductive properties. However, they do lack osteoinductive properties that are associated with allograft or autograft bone [15,16]. The ceramic grafts are dependent on the remaining bone for their successful outcome [16]. Researchers have tried to provide this capacity to ceramic implants by adding bone marrow cells [17] in addition to osteoinductive proteins [15] to improve their osteoinductive capabilities. Both patients received BMP and bone marrow aspirate, which could help explain the solid bony fusion, but there is not enough consistent evidence. Future, high-quality studies will need to test this theory.
The lack of subsidence, migration and implant fracture/failure was expected due to the potential theoretical advantages of the the Valeo® (Valeo® TL VBR Lumbar Spacer, Amedica Corporation, Salt Lake City, UT) silicon nitride ceramic implant, and that neither patient had osteoporosis. No subsidence and no change in implant placement is clinically relevant as it importantly preserves the desired maintenance of the interbody disc space restoration and sagittal alignment. Unlike titanium, PEEK and CFRP cages, which have been known to subside [5], slip and migrate [5,11], as there is no ability for bony ingrowth to the native vertebral bodies [4,18,19], ceramics are thought to be safe, and believed to be associated with a high bone-bonding capacity. The proprietary nature of the ceramic, composed of medical grade silicon nitride (Si3N4) with a special textured cortico-cancellous structured surface, has been suggested to allow bony ingrowth to occur to the corresponding vertebral bodies. Additionally, the ceramic implant has been marketed as a dense, hydrophilic construct for load bearing that is strong and can exhibit high fracture toughness. The concern has lied in the fact that few peer-reviewed publications have been available to confirm these theories. In the present case report, the patients demonstrated bone ingrowth to the implant was able to occur at the same rate as the interbody fusion, which is likely why the implant did not subside or migrate in either patient.
Since the interbody spacer is one of the key players of a successful fusion, our results suggest that a ceramic implant may be a better option in a TLIF procedure than other standardly used implants made out of titanium, PEEK or CFRP. Future prospective, comparative studies with large sample sizes will need to confirm the present results and anecdotal theories.
Historically, sophisticated imaging was challenging with a titanium interbody implant as the titanium results in significant image distortion due to metallic scatter and distortion, which made intra op placement and fusion determination challenging. An abstract presented at the 8th Annual Spine Arthroplasty Society Global Symposium on Motion Preservation Technology compared the clinical visibility of cylindrical shaped specimens composed of cobalt chromium, polyetherketone (PEEK), titanium and silicon nitride ceramic (Si3N4) [20]. As compared to the other commercially available specimens, the study concluded that silicon nitride ceramic implants may be easier to follow postoperatively due to lack of distortion under magnetic resonance and lack of scattering under computed tomography.
Two major limitations of this paper are obvious. First, this was a case study paper; thus, only two non-osteoporotic patients were reviewed. Second, there were no comparative counterparts for these two patients. Nonetheless, these cases are unique and will aid future work.
Conclusion – Silicon Nitride Ceramic Implants
Both patients demonstrated solid bony fusion on dynamic x-rays and CT scans at 1-year follow up, which may be due to the unique ceramic qualities and better imaging compatibility of the Valeo® silicon nitride ceramic implant. In addition, in these two non-osteoporotic patients, there was no evidence of subsidence, migration or implant fracture/ failure. Our findings suggest that the ceramic implant may provide an attractive alternative for use in a TLIF procedure as compared to standard titanium, PEEK or CFRP implants. The need for a larger prospective study to determine the efficacy of the use of Valeo® is warranted.
Funding
No funding was provided or received for this research or any other form of support from industry.
Conflict of Interests
The following authors acknowledge a potential conflict of interest: JAY, royalties, stock options from Amedica Corporation, Salt Lake City, UT. BSB, board member, Amedica Corporation, Salt Lake City, UT.
For More Information Please Reach Out on the Contact Form.
References
- Kornblum MB, Fischgrund JS, Herkowitz HN, Abraham DA, Berkower DL, et al. (2004) Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine (Phila Pa 1976) 29: 726-33.
- Blume HG (1985) Unilateral posterior lumbar interbody fusion: simplified dowel technique. Clin Orthop Rela Res 193: 75-84.
- Harms J, Rolinger H (1982) Die operative Behandlung der Spondylolisthese durch dorsal Aufrichtung und ventral Verblockung. Z Orthop Ihre Grenzgeb 120: 342-347.
- Aoki Y, Yamagata M, Nakajima F, Ikeda Y (2008) Takahashi K Posterior migration of fusion cages in degenerative lumbar disease treated with transforaminal lumbar interbody fusion. Spine 34: E54-E58.
- Cole CD, McCall TD, Schmidt MH, Dailey AT (2009) Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskelet Med 2:118-126.
- Lauber S, Schulte TL, Liljenqvist U, Halm H, Hackenberg L (2006) Clinical and radiological 2-4-year results of transforaminal lumbar interbody fusion in degenerative and isthmic spondylolisthesis Grade 1 and 2. Spine 31: 1693- 1698.
- Lowe TG, Tahernia AD, O’Brien MF, Smith DAB (2002) Unilateral transforaminal posterior lumbar interbody fusion (TLIF): indications, technique and 2-year results. J of Spinal Disord and Tech 15: 31-38.
- Mummaneni PV, Pan J, Haid RW, Rodts GE (2004) Contribution of recombinant human bone morphogenetic protein-2 to the rapid creation of interbody fusion when used in transforaminal lumbar interbody fusion: preliminary report. J Neurosurg Spine 1: 19-23.
- Foley KT, Holly LT, Schwender JD (2003) Minimally invasive lumbar fusion. Spine 28: S26-S35.
- McAfee PC, DeVine JG, Chaput CD, Prybis BG, Fedder IL, et al. (2005) The indications for interbody fusion cages in the treatment of spondylolisthesis: analysis of 120 cases. Spine 30: S60-S65.
- Collis JS (1985) Total disc replacement: a modified posterior lumbar interbody fusion. Report of 750 cases. Clin Orthop Relat Res 193: 64-67.
- Bridwell KH, Lenke LG, McEnery KW, Baldus C, Blanke K (1995) Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine 20: 1410-1418.
- Brantigan JW, Steffee AD, Lewis ML, Quinn LM, Persenaire JM (2000) Lumbar interbody fusion using the Brantigan I/F cage for posterior lumbar interbody fusion and the variable pedicle screw placement system: two-year results from a Food and Drug Administration investigational device exemption clinical trial. Spine 25: 1437-1446.
- Marchi L, Abdala N, Oliveira L, Amaral R, Coutinho E, et al. (2013) Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. J Neurosurg Spine 19: 110-8.
- Delécrin J, Takahashi S, Gouin F, Passuti N (2000) A synthetic porous ceramic as a bone graft substitute in the surgical management of scoliosis. Spine 25: 563-569.
- Moore WR, Graves SE, Bain GI (2001) Synthetic bone graft substitutes. ANZ J Surg 71: 354-361.
- Grundel RE, Chapman MW, Yee T, Moore DC (1991) Autogenic bone marrow and porous biphasic calcium phosphate ceramic for segmental bone defects in the canine ulna. Clin Orthop 266: 244-258.
- Nguyen HV, Akbarnia BA, van Dam BE, Raiszadeh K, Bagheri R, et al. (2006) Anterior exposure of the spine for removal of lumbar interbody devices and implants. Spine 31: 2449-2453.
- Taneichi H, Suda K Kajino T, Matsumura A, Moridaira H, Kaneda K (2006) Unilateral transforaminal lumbar interbody fusion and bilateral anteriorcolumn fixation with two Brantigan I/F cages per level: clinical outcomes during a minimum 2-year follow-up period. J Neurosurg Spine 4: 198-205.
- Anderson M, Bernero J, Brodke D (2008) Medical imaging characteristics of silicon nitride ceramic: a new material for spinal arthroplasty implants. Abstract presented at the 8th Annual Spine Arthroplasty Society Global Symposium on Motion Preservation Technology, Miami, FL.