3D Printing of Medical Implant Devices with FleX-SN PEEK
By leveraging the unique properties of silicon nitride and the transformative power of 3D printing, SINTX is able to create high-performance 3D printed medical implant devices with exceptional biocompatibility, resulting in high rates of acceptance and long-term implant success.
3D Printing Technology in Medicine
Three-dimensional (3D) printing refers to several manufacturing technologies that generate a physical model from digital information. Every year, 3D printing (also called additive manufacturing or additive layer manufacturing) offers numerous applications in the healthcare field, helping to save and improve lives in ways never imagined up to now.
This technology allows medical device manufacturers, such as SINTX, a high degree of intricacy and customization. With 3D printing, implantable medical devices can be created to match very complex structures within a patient’s anatomy. For the FleX-SN PEEK, SINTX is using polymer 3D printing technology.
SINTX’s Outlook as a Medical Implant Manufacturer
With SINTX’s recent acquisition of polymer processing equipment (e.g., twin-screw extruder and ancillary equipment, plastic shredder/recycler, injection molder, and medical-grade 3D printer), we are now able to develop novel composite filaments for use in fabricating next-generation medical implants via additive manufacturing with the FleX-SN PEEK material.
Newly acquired polymer processing equipment:

With this equipment, we intend to utilize our expertise in ceramics and polymer processing with our growing knowledge of 3D printing medical devices to produce complex, novel implant designs of varying silicon nitride compositions. Our medical-grade 3D printer will allow us to fabricate parts with textures and porosities, features that cannot be produced by any other manufacturing method.
Our development of composite filament will allow us to produce 3D printed medical implants with enticing characteristics like the decreased chance of infection, biocompatibility, favorable mechanical properties, and osteogenic properties to further increase the body’s likelihood of acceptance of the implant.
Silicon Nitride – the Ideal Biomaterial for 3D Printed Medical Implants
Silicon nitride is a ceramic material that is known for its high strength and excellent biocompatibility making it the ideal biomaterial material for 3D printed implants which need to be able to withstand the mechanical stresses of the body.
When used to make spinal fusion implants, silicon nitride has the flexibility of a dense, porous architecture that can mimic the cortical cancellous structure of bone. Silicon nitride is biocompatible, bioactive, and has shown bacterial resistance and superb bone affinity. With >33,000 human spine implantations over 10 years, and less than 25 reported adverse events, silicon nitride has a good safety record. Silicon nitride can be polished to a smooth and wear-resistant surface for articulating applications, such as bearings for hip and knee replacements.
Silicon Nitride Based Polymers for Medical Devices
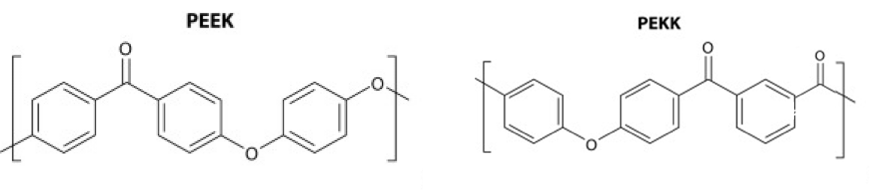
Composite materials have long been used to exploit the characteristics of different materials by combining them. The composites we are making at SINTX consist of our silicon nitride powder in a matrix of PEEK (Polyether ether ketone), which is the FleX-SN PEEK product, or PEKK (Polyether ketone ketone) which belong to the family of PAEK (Polyaryletherketone) polymers.
PAEK polymers are semi-crystalline thermoplastics with high temperature, mechanical, and chemical resistance properties. Utilizing these composite filaments in the 3D printing of medical devices, we are now able to combine silicon nitride’s demonstrated hydrophilicity, radiopacity (favorable imaging via x-ray detection), antipathogenic, and osteogenic properties with the mechanical properties of PEEK and PEKK which are similar to that of human bone for use in medical implants.
PEEK & PEKK Monomers:
Medical Applications of 3D Printing with Silicon Nitride
3D printing of medical devices has a variety of applications, particularly in the manufacture of custom 3D printed medical implants for use in spinal dental, and maxillofacial surgery. Using silicon nitride (Si3N4) for these implants can give them antibacterial and osteogenic (promoting bone growth) properties:
Spinal Implants
With over 40,000 human spine implantations over 10 years and a very low percentage of reported adverse events, FleX SN-MC2 “silicon nitride” has an excellent safety record. It is biocompatible, bioactive, and has shown bacterial resistance and superb bone affinity.
Dental Implants
As a dental implant material, FleX SN-MC2 “silicon nitride” has been thoroughly tested to ISO 10993-01 standards and shown to be highly biocompatible. Silicon nitride ceramic is metal-free, like all ceramics, and free of corrosion, galvanism, and electronic disturbances, offering the latest technology in dental implants.
Craniomaxillofacial Implants
When used in implants for CMF applications, silicon nitride has the potential to accelerate bone healing, eliminate metal toxicity, and enhance radiographic imaging. Because it’s antipathogenic, silicon nitride can also decrease infection rates and ease the healthcare burden associated with failed implants.
Foot Osteotomy & Fusion
Silicon nitride has the flexibility of a dense, porous, or combined architecture that can mimic the cortical-cancellous structure of bone. This characteristic combined with numerous other benefits, such as antibacterial resistance, make silicon nitride a strong consideration as an implant material in this application.
In Conclusion
Silicon nitride has a fortuitous combination of strength, toughness, wear resistance, biocompatibility, bioactivity, bone integration, structural stability, corrosion resistance, and ease of imaging; all of which are desired properties in medical implants.
By harnessing the unique properties of medical-grade silicon nitride and the limitless possibilities of 3D printing, SINTX and other medical device manufacturers can produce highly complex, high-performance 3D printed implants that achieve exceptional rates of acceptance and improve patient outcomes.
OEM Partnerships with SINTX
Since its inception, SINTX Technologies has been focused on medical-grade silicon nitride.
With years of research and expertise, we’re experts in silicon nitride application development. Our unrivaled in-house manufacturing capabilities allow for intricate designs and shapes that can be rapidly developed, prototyped, and tested across a wide variety of medical applications.
We invite you to Contact SINTX Technologies to see how silicon nitride might be beneficial to your patients, practice, or medical device manufacturing facility. Get started on your custom 3D printed medical devices.
References:
- https://sintx.com/wp-content/uploads/2020/09/412060-Silicon-Nitride-Properties-and-Specifications-A.pdf
- https://www.washington.edu/news/2013/05/14/engineered-biomaterial-could-improve-success-of-medical-implants/
- https://www.fda.gov/medical-devices/products-and-medical-procedures/3d-printing-medical-devices
